Types of Aluminum materials:
- Pure Aluminum
- Aluminum-Copper Alloys
- Aluminum-Manganese Alloys
- Aluminum-Silicon Alloys
- Aluminum-Magnesium Alloys
- Aluminum-Magnesium-Silicon Alloys
- Specialty Alloys
Aluminum alloy is a metallic material formed by adding one or more alloying elements such as copper, magnesium, silicon, zinc, manganese, etc. to aluminum (Al) as the base element.
As the most widely used non-ferrous metal structural material in industry, its usage volume is second only to steel.
It features light weight, high strength, corrosion resistance and other characteristics, and is one of the most popular alloy materials in modern industry.
The core definition and composition of aluminum alloys
The proportion of aluminium is usually ≥90%, and the rest are alloying elements:
- Main elements
- Trace elements
- Alloying principle
Main elements
Copper (Cu), magnesium (Mg), silicon (Si), zinc (Zn), manganese (Mn), used to enhance strength, hardness or heat resistance.
Trace elements
Nickel (Ni), titanium (Ti), chromium (Cr), lithium (Li), etc., are used to refine grains or enhance specific properties.
Alloying principle
Through mechanisms such as solid solution strengthening and precipitation hardening, it overcomes the defect of low strength of pure aluminum tensile strength about 90MPa.

Aluminum materials
- Pure Aluminum
- Aluminum-Copper Alloys
- Aluminum-Manganese Alloys
- Aluminum-Silicon Alloys
- Aluminum-Magnesium Alloys
- Aluminum-Magnesium-Silicon Alloys
- Specialty Alloys
Pure Aluminum
Composition: Al ≥99.00% (e.g., 1050, 1070, 1100).
Properties:High conductivity (61% IACS) and thermal conductivity.
Excellent corrosion resistance due to natural oxide layer.
Low strength (σb: 80–110 MPa), high plasticity.
Applications:Electrical cables, heat sinks, food packaging, and decorative panels.

Aluminum-Copper Alloys
Composition: Cu (3–5%) as main alloying element.
Properties:High strength (σb >400 MPa after heat treatment).
Poor corrosion resistance; prone to intergranular corrosion.
Applications:Aircraft structures (wing beams, landing gear), high-stress automotive parts.

Aluminum-Manganese Alloys
Composition: Mn (1.0–1.5%).
Properties:Moderate strength (σb: 200–300 MPa), excellent formability and weldability.
Superior corrosion resistance in humid/chemical environments.
Applications:Building facades, beverage cans, heat exchangers, and vehicle bodies.
Aluminum-Silicon Alloys
Composition: Si (4.5–13.5%) (e.g., 4032, 4047).
Properties:Low thermal expansion, high wear resistance, and excellent fluidity for casting.
Heat resistance up to 400°C.
Applications:Engine pistons, welding wire, and electronic encapsulation.

Aluminum-Magnesium Alloys
Composition: Mg (3–5%) (e.g., 5052, 5083).
Properties:Exceptional corrosion resistance in marine environments.
Medium strength (σb: 200–300 MPa), lightweight (density: 2.68 g/cm³).
Applications:Ship hulls, offshore platforms, pressure vessels, and automotive fuel tanks.
Aluminum-Magnesium-Silicon Alloys
Composition: Mg + Si forming Mg₂Si precipitates (e.g., 6061, 6063).
Properties:Heat-treatable for high strength (σb: 240–280 MPa after aging).
Good machinability and surface finish for anodizing.
Applications:Architectural profiles, bicycle frames, and automotive chassis.

Aluminum-Zinc-Magnesium Alloys
Composition: Zn + Mg + Cu (e.g., 7075, 7050).
Properties:Ultra-high strength (σb >500 MPa), excellent fatigue resistance.
Poor corrosion resistance; requires coatings for harsh environments.
Applications:Aircraft fuselages, rocket components, and high-stress sports equipment.
Specialty Alloys
- Lithium-Aluminum Alloys
- Casting Alloys
Lithium-Aluminum Alloys
Properties: Reduced density (−3% per 1% Li), high stiffness.
Applications: Aerospace structures to reduce weight.
Casting Alloys
Al-Si (e.g., A356): Excellent castability, used for engine blocks.
Al-Mg (e.g., 508.3): High strength and corrosion resistance for marine components.
Processing: Suited for die-casting, sand casting, and low-pressure molding.

Aluminum Composites
Reinforcements: Carbon fiber, SiC particles.
Properties: 20–50% higher strength than base alloys.
Applications: Brake rotors, aerospace brackets.

What are the specific influence mechanisms of different alloying elements in aluminum alloys on the material properties?
- The influence mechanism of copper (Cu)
- The influence mechanism of magnesium (Mg)
- The influence mechanism of zinc (Zn)
The specific influence mechanisms of different alloying elements such as copper, magnesium.
And zinc in aluminum alloys on material properties can be analyzed from multiple aspects.
Including strengthening mechanisms, phase transformation, solid solution strengthening, grain refinement, and stress corrosion tendency, etc.
The influence mechanism of copper (Cu)
Copper in aluminum alloys mainly enhances the strength and hardness of the alloys through solid solution strengthening and aging strengthening.
The addition of copper can enhance the tensile strength and hardness of aluminum alloys, but it will reduce their corrosion resistance and plasticity.
The copper content is usually between 2.5% and 5%, and the strengthening effect is the best when the content is between 4% and 6.8%.
The addition of copper can also promote precipitation hardening and enhance the strength of the alloy.
The influence mechanism of magnesium (Mg)
Magnesium is an important strengthening element in aluminum alloys, mainly enhancing the strength of alloys through solid solution strengthening and precipitation strengthening.
The addition of magnesium can significantly enhance the tensile strength and machinability of aluminum alloys, but excessive magnesium can lead to increased brittleness.
Magnesium combines with silicon to form the Mg2Si phase, further strengthening the alloy.
The addition of magnesium can also enhance the weldability and corrosion resistance of aluminum alloys.

The influence mechanism of zinc (Zn)
Zinc in aluminum alloys mainly enhances the strength of the alloys through solid solution strengthening and precipitation strengthening.
Zinc alone added to aluminium has a limited effect on enhancing strength, but when combined with magnesium (such as in Al-Zn-Mg alloys), it can significantly increase strength.
However, there is a tendency for stress corrosion cracking (SCC).
The addition of zinc can also improve the heat treatment performance of aluminum alloys, but excessive zinc may lead to stress corrosion cracking.
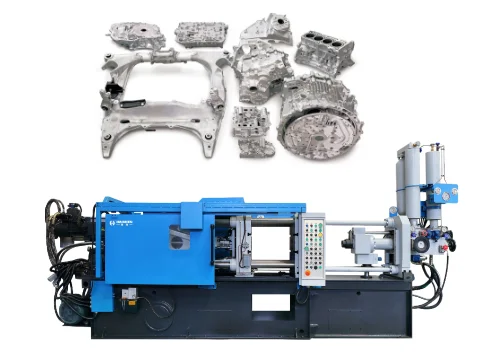
Haichen’s aluminum die-casting
Ultimately, which metal forming process you choose depends on your budget, the purpose of the aluminum part, and the number of parts you need to produce.
Die casting can be a more expensive process, but it’s worth it if your design is very complex and requires a lot of parts.
Haichen provides professional aluminum die casting services, including professional mold design, ensuring that the molten metal fills all parts of the mold.
Our die-casting machines, cells and solutions are tailored to your needs. With integrated control systems you get a comprehensive overview of your cell. This helps you to improve the die-casting process and to keep an eye on production.
And ISO 9001:2015 certified production facilities, offering expert manufacturing, superior quality control, and consistent performance.
Our solutions for aluminum and magnesium die-casting are tailored for your foundry to increase productivity and efficiency.
We also run a global network of technical experts and support engineers who can help you, from planning to installation and support.
If you want to learn about other aluminum fabrication options, check out our article on die casting vs. aluminum extrusion. Please feel free to contact us.




